Abstract
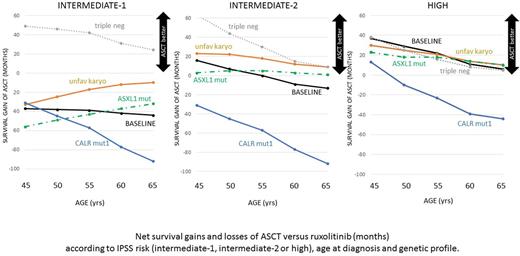
Allogeneic transplantation (ASCT) offers a chance of cure for younger patients with myelofibrosis (MF) and non-relapse mortality (NRM) has progressively declined thus making transplant feasible also in older patients. Since JAK2 inhibitors recently showed to ameliorate survival of intermediate-2 and high-risk patients, the selection of candidates for ASCT has become particularly complex. The large number of predictive variables influencing the decision to transplant MF patients makes exhaustive multivariate analyses unfeasible. Moreover, no case-control study could yet include a large portion of patients assigned to ruxolitinib. Therefore, we implemented a decision model simulating a cohort of 10,000 55 year-old patients newly diagnosed intermediate 2 MF: patients were assigned either to ASCT or to ruxolitinib. Patients assigned to ASCT were allowed to move monthly among 4 Markov health states (pre-transplant, post-transplant, relapse and death) according to pooled data from a systematic review of transplant reports published (EMBASE) from January 2010 to May 2017 and reporting at least 30 patients. We retrieved 50 publications (4 prospective studies) reporting 4,341 MF patients receiving ASCT mostly (62%) from matched donors, mostly after the year 2000. Median age at transplant was 55 years and median time elapsed from diagnosis was 21 months. IPSS at transplant was mainly intermediate-2 (40%) or high (21%), however, 216 ASCT were performed after blast transformation. At a median follow-up of 38 months, 2-year non-relapse mortality (NRM) was 25% (95%CI: 21.4-28.6%) and cumulative incidence of relapse 22% (95%CI: 17.5-26.5%). On the other arm of the decision model, patients assigned to ruxolitinib had a 0.0105 monthly chance of moving from the alive to the dead state, according to the survival rates reported by COMFORT trials (Vannucchi'15, Verstovsek '17). The same probability of death was assigned to patients waiting for ASCT. Based on the above input data, the model outputs confirmed an appropriate calibration: 5-year cumulative NRM was 25% and 5-year survival in ruxolitinib-treated patients was 59%. The above model forecast 101 versus 99 mean survival from diagnosis if ASCT is included into the treatment pathway. However, very large confidence intervals surrounded the above estimates, therefore, sensitivity analysis explored the impact of different age, IPSS risk and molecular profile, based on the hazard ratios reported by multivariate analyses (Gangat'11, Guglielmelli'17, Palandri'17, Kroger'17, Panagiota'14, Bregante'16, Ditchowasky'12). Despite the long survival yield of ruxolitinib, ASCT provided a survival gain higher than 6 months in intermediate-2 patients younger than 50 years and in high risk patients younger than 65 years. On converse, patients with intermediate-1 risk (- 39 months) or carrying CALR mut 1 (-41 months) were expected to achieve a shorter survival with ASCT. The model allowed to explore complex combinations of clinical and genetic profiles: results are reported in the Figure. ASCT advantage over ruxolitinib was questioned in high-risk older patients harboring CALR mut1, while ASCT was expected to provide a longer survival in intermediate-1 patients lacking any of the three funding mutations. Despite its simplicity, the present decision model has the potential to test several clinical scenarios, depending on the clinical response to ruxolitinib, the presence of multiple detrimental mutations or the availability of alternative donors. Still the model has some limitations mainly related to the assumed multiplicative interactions among predictive variables and to the assumed exponential shape of survival curves. Secondly, the model was calibrated as to project the same five-year survival as ruxolitinib-treated patients of COMFORT studies, but longer follow-up survival data are still missing. Moreover, survival gains of a few months should be carefully balanced against quality of life detriments imposed by ASCT. Nevertheless, results are consistent with recent consensus recommendations and, as a larger portion of patient is tested for funding and non-funding mutations, integration and validation of the model will be possible by retrospective databases.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal